Abstract
Background Treatment of multiple myeloma (MM) has advanced significantly in the last 20 years, resulting in improved survival. For patients with newly diagnosed MM (NDMM) who are fit with no comorbidities, currently recommended treatment includes induction with triplets such as bortezomib, lenalidomide, and dexamethasone (VRd), followed by high-dose chemotherapy (HDT) and autologous stem cell transplantation (ASCT), and maintenance therapy using predominantly immunomodulatory agents. Patients who are elderly or ineligible for HDT/ASCT receive multiple drug combination therapies such as VRd or daratumumab with Rd. Despite the availability of these combinations, induction therapy does not induce complete remission with minimal residual disease negativity in all patients, and triplet- or quadruplet-based regimens are expensive and associated with enhanced toxicities including to stem cells. Moreover, MM remains incurable and new therapies with alternative mechanisms may improve or maintain the safety profile while increasing or deepening responses for longer periods.
B-cell maturation antigen (BCMA) is a cell surface protein expressed on malignant plasma cells (Lee, et al. Br J Haematol. 2016;176:911-22). Notably, BCMA is detected only at low levels on normal plasma cells, activated B cells, and plasmacytoid dendritic cells, making it an attractive therapeutic target for MM.
REGN5458 is a BCMA×CD3 bispecific antibody capable of binding to BCMA on MM cells and CD3 on T cells, inducing T-cell mediated cytotoxicity of MM cells. REGN5458 monotherapy has demonstrated early, deep, and durable responses with a manageable safety and tolerability profile in patients with relapsed/refractory (R/R) MM (NCT03761108; Zonder, et al. EHA 2022. Abstract S189) with a response rate of 75% among patients treated at the 200-800 mg dose levels. Cytokine release syndrome was reported in 38% of patients and was mostly Grade 1, with no Grade ≥3 events. Given the single agent efficacy and tolerability of REGN5458 in heavily pre-treated patients with R/R MM, its use in patients with NDMM (who are thought to potentially have improved T-cell fitness versus R/R patients) is hypothesized to have enhanced therapeutic benefit.
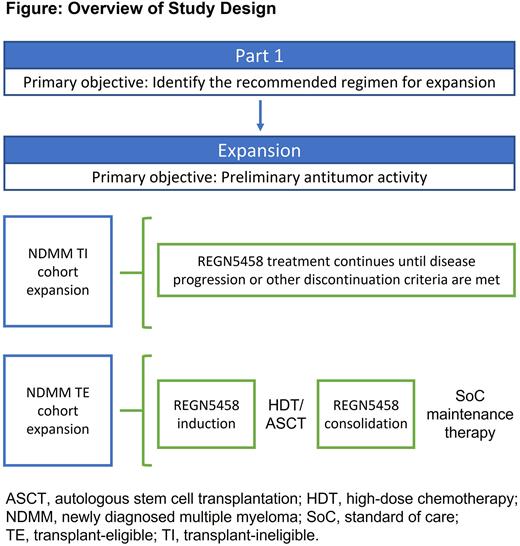
Study Design and Methods This open-label, Phase II study aims to assess the safety, tolerability, pharmacokinetics (PK), and preliminary efficacy of REGN5458 monotherapy in patients with NDMM who are eligible or ineligible for HDT/ASCT. Transplant-eligible patients will receive REGN5458 induction, HDT/ASCT, followed by REGN5458 consolidation, and standard of care maintenance therapy. Transplant-ineligible patients will receive REGN5458 until disease progression or other discontinuation criteria are met (Figure). The primary objectives during part 1 are to assess safety and tolerability and to determine a recommended regimen for expansion. The objective for dose expansion is to assess preliminary antitumor activity. Secondary objectives are to evaluate the PK properties and immunogenicity of REGN5458 as well as soluble BCMA concentrations in serum at baseline and over time.
The primary endpoints for part 1 are: the incidence of dose-limiting toxicities (DLTs) from the first dose of study drug to the end of the DLT observation period; the incidence and severity of treatment-emergent adverse events and adverse events of special interest with REGN5458 through study completion; for expansion: the proportion of patients with a very good partial response or better as determined by the investigator using International Myeloma Working Group response criteria; and the proportion of patients achieving minimal residual disease-negative status (at 10-5).
Patients will have a window of opportunity to benefit from treatment; patients who do not meet the specified efficacy thresholds will transition to standard of care therapies. Approximately 92 patients are planned for this study. Eligibility criteria include age ≥18 years; ECOG PS 0-2; histologically or cytologically confirmed symptomatic MM; measurable disease; no prior therapy for MM, except radiation on an emergency/palliative basis or 1 month of single-agent corticosteroid if the patient still has measurable disease; and adequate bone marrow reserves, hepatic, renal, and cardiac function.
The study will take place in approximately 20 sites located in North America and Europe. There are no formal hypothesis tests for this study.
Disclosures
Ferreri:Affimed: Current equity holder in publicly-traded company; Sanofi: Membership on an entity's Board of Directors or advisory committees. Quatela:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Aina:Regeneron Pharmaceuticals, Inc.: Consultancy, Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Roy:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Boyapati:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Rodriguez Lorenc:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Kroog:Regeneron Pharmaceuticals, Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Orlowski:Abbvie, BioTheryX, Inc., Bristol-Myers Squibb, Janssen Biotech, Karyopharm Therapeutics, Inc., Meridian Therapeutics, Monte Rosa Therapeutics, Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, Inc., Sanofi-Aventis, and Takeda Pharmaceutic: Honoraria, Membership on an entity's Board of Directors or advisory committees; CARsgen Therapeutics, Celgene/Bristol Myers Squibb, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Research Funding; Asylia Therapeutics, Inc.: Current equity holder in private company; Asylia Therapeutics, Inc., BioTheryX, Inc., Heidelberg Pharma, Inc.: Research Funding.
OffLabel Disclosure:
Off-Label Drug Purpose: The abstract describes the study design and methods of an open-label, Phase II study assessing REGN5458 monotherapy in patients with newly diagnosed multiple myeloma.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal